Epigenetics and drug addiction: predisposition, chronic use, relapse and treatment
by Dr. Sanne Janssen
For those who have not experienced drug addiction, it can be easy to view the disease as a choice. “Why did he choose to use cocaine? Why did she not choose to stop using?” But of course, it isn’t that simple. Research shows that genes—and their epigenetic regulation—play an important role in shaping susceptibility to addiction.
Drug addiction develops in several stages: initiation of drug use, intermittent to regular use, and finally, addiction, which is often followed by cycles of withdrawal and relapse. The stages of drug addiction are affected by a combination of non-reversible genetic and reversible epigenetic factors.
To gain a better understanding of epigenetics in drug addiction, researchers use animal models that allow for molecular studies in the brain areas targeted by drugs, which are collectively known as the reward system.
Here, we focus on altered histone acetylation and methylation. You can read more about drug addiction-related changes in DNA methylation here and here.
Predisposition and vulnerability
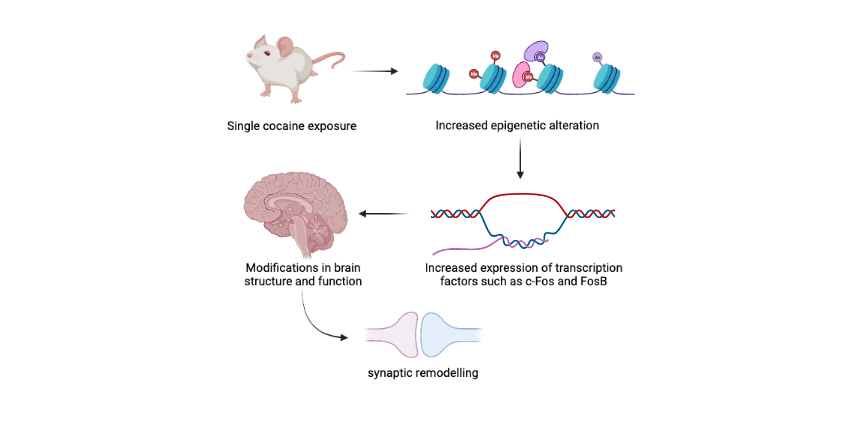
Predisposition to developing drug addiction depends both on a person’s genetic and epigenetic state. In other words, in addition to genetic mutations or gene alleles that modulate an individual’s susceptibility to addiction, the epigenetic landscape can also render a person more or less predisposed to developing drug addiction. Indeed, studies show that short-term drug use results in alterations of the epigenetic landscape.
For example, a single exposure to cocaine triggers an immediate increase in acetylation of histone H4 at the promoter of the c-Fos gene in mice, leading to increased expression of this important transcription factor. Similarly, short-term exposure to cocaine leads to histone H4 acetylation at the FosB promoter in mice. This results in increased expression of FosB, a transcription factor that belongs to the same family as c-Fos. Gene expression changes such as these bring about modifications in brain structure and function, including synaptic remodeling, that could promote vulnerability to drug addiction.
While we know that single drug exposure may increase vulnerability to drug addiction by inducing immediate changes to the epigenetic landscape, whether or not epigenetic factors influence drug addiction vulnerability for people without prior drug use remains unknown.
This is mainly due to the challenges that come with studying humans, as the environment continuously influences our epigenetic landscape; we cannot know or predict if someone will develop a substance use disorder in the future. These caveats in human studies of epigenetics in relation to drug use are one of the reasons why researchers turn to animal models.
Chronic drug use and behavioral response
Similar to a single drug use, chronic drug use leads to epigenetic and gene expression changes in the brain’s reward system; however, in contrast to a single exposure, chronic drug exposure-mediated changes contribute to life-long drug-related behaviour.
One of the best characterized epigenetic alterations due to chronic drug exposure in rodents is an increase in global acetylation, including of histones H3 and H4, resulting from both increased histone acetylation and decreased activity of the enzymes that deacetylase histones (i.e., histone deacetylases, HDACs).
In general, histone acetylation is associated with open chromatin, making genes more accessible to the transcriptional machinery. The increase in histone acetylation due to chronic drug exposure in rodents is associated with increased expression of genes including Bdnf, Cdk5 and delta FosB.
One mechanism by which delta FosB appears to promote drug exposure-related behaviour is by binding to the gene promoter of the opioid peptide dynorphin, resulting in downregulation of the dynorphin gene Pdyn and consequently increased reward sensitivity. In addition, FosB overexpression in the nucleus accumbens reduces sensitivity to the analgesic effects of morphine and cocaine exposure, while increasing the sensitivity to reward effects, possibly via changing Pdyn expression.
Interestingly, another study revealed that upon chronic amphetamine exposure, delta FosB recruits HDAC1 to the promoter of c-Fos, removing acetylation and reducing c-Fos gene expression. This may be important for long-term drug exposure-related behaviour, as expression of c-Fos decreases memory of the reinforcing effects of cocaine in mice.
Thus, chronic drug use leads to epigenetic modifications that altered gene expression, including of FosB, which directly and indirectly contributes to long-term drug-related behavior.
Withdrawal and relapse
A major challenge in treating drug addiction is the tendency to relapse. One underlying cause of relapse is changes in the brain during the period of withdrawal, including alterations in epigenetic factors and modifications that can persist for a long time after the last drug exposure. However, changes in gene expression don’t always have a negative effect on withdrawal or relapse.
For instance, the Nr4a1 gene appears to suppress cocaine-induced behavior during withdrawal in mice. Upon cocaine exposure, the promoter of Nr4a1 gains histone modifications (H3K27ac and H3K4me3) that are permissive to gene transcription, leading to increased Nr4a1 expression in the nucleus accumbens where it regulates other genes involved in dopamine metabolism and suppresses drug-related behaviour. Interestingly, while this study indicates that Nr4a1 is important in relapse behaviour, it also suggests that it may not play a role in vulnerability to drug addiction.
Thus, similar to chronic drug use, epigenetic alterations and related gene expression changes that occur during the period of withdrawal affect the tendency to relapse. In addition, gene expression changes induced by cocaine exposure can persist during withdrawal. How this persistence affects relapse behaviour and if epigenetic modifications are also retained during withdrawal remains to be determined.
Epigenetic therapeutics
Epigenetic alterations are reversible, which makes them interesting therapeutic targets. As one of the best characterised effects of drug exposure is on histone acetylation, many studies have focused on the enzymes that acetylate or deacetylate histones (i.e., HDACs and HATs).
For example, deletion of the histone acetyltransferase CBP in the nucleus accumbens of mice was found to reduce histone H3 acetylation and c-Fos expression in response to cocaine administration. More importantly, loss of CBP impaired drug-seeking behavior, indicating that CBP-mediated histone acetylation has a negative influence on drug-motivated behaviour. On the other hand, HDAC inhibition was shown to increase histone H3 acetylation, c-Fos expression and enhance drug-related behaviour upon short-term cocaine administration.
Unfortunately, the relationship between acetylation status and drug use-related behaviour as observed in rodents is not that straight forward. For example, in contrast to the findings described above, treatment with HDAC inhibitors during cocaine withdrawal in mice or rats was found to facilitate withdrawal by preventing drug-induced behavioural changes and reducing the risk on relapse, even though H3 acetylation is increased.
Another study in rats showed that HDAC inhibition did not change heroin self-administration 12 or 24 hours after treatment. In addition, heroine-seeking behaviour was increased when the HDAC inhibitor was administered during withdrawal 12 hours before making heroin available.
Besides the effect of HDAC inhibition on histone acetylation, a specific HDAC inhibitor that blocks HDAC1 also induces specific methylation of histone H3 called H3K9me2, a histone modification that facilitates repression of gene expression. This finding raises an important point: targeting one epigenetic modification, like histone acetylation, often results in changes to another epigenetic modification, since there is crosstalk between different types of histone modifications as well as between histone modifications and DNA methylation.
It is important to note that the studies described here are done in different types of rodents, administrating different HDAC inhibitors for different time periods during different stages of drug exposure, and using different experimental procedures to assess their effect, which likely explains some of the contrasting findings.
Thus, epigenetic alterations acquired through drug use may be reversible and therapeutics that target epigenetic modifications are being tested in rodent models. Unfortunately, due to conflicting results, more work is needed to identify which treatments would be best suited at different stages of addiction.
Take home messages
-
We have very little control over our genetic and epigenetic landscape
-
Our genetics and epigenetics can predispose us to drug use
-
Drug use itself triggers epigenetic changes that can stimulate addiction, and later relapse
-
Despite conflicting evidence, therapies that target our epigenetic landscape are promising candidates for the future of drug use treatment
Learn More
- Learn more about drug addiction-related changes in DNA methylation here and here.
- National Institute on Drug Abuse: Genetics and Epigenetics of Addiction.
- National Institute on Drug Abuse: Words Matter – Terms to use and avoid when talking about addiction.
We would like to thank Dr. Rosemary Bagot from McGill University for her expert review of this article.
Image created in www.BioRender.com