Cat fur and color blindness: what X-chromosome inactivation teaches us about health.
What do cat fur and color blindness have in common? The answer lies within epigenetics. Let’s talk about a mechanism called X-chromosome inactivation, and its implications in health and disease.
X and Y: what are sex chromosomes?
First, we need to understand what chromosomes are. The nucleus of our cells contains the genetic information that we inherit from our parents. This genetic material is DNA and contains thousands of genes which control many of our physical traits, such as height, blood types or eye color. And just like an encyclopedia can be divided into several volumes, our DNA is divided amongst chromosomes. Humans have 23 pairs of chromosomes – one set being inherited from each parent.
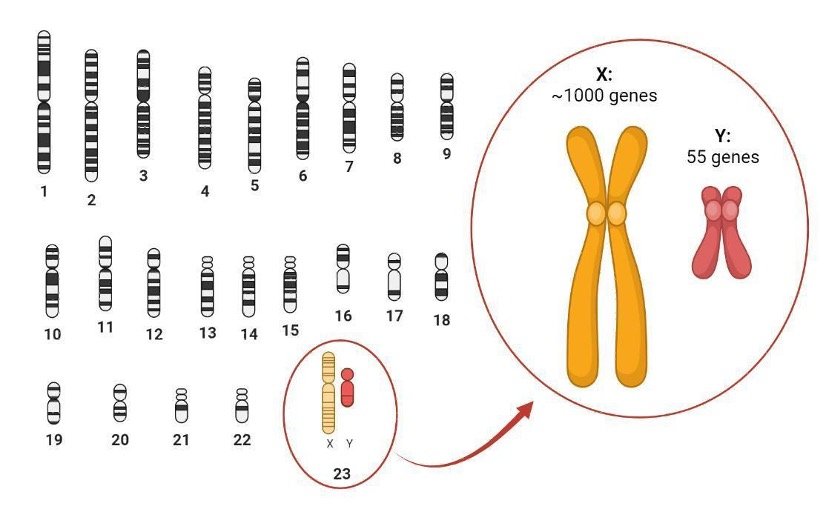
One pair of chromosomes stands out because it comprises two types of chromosomes, X and Y, which vary widely in size and content (Figure 1). Biological sex is typically determined by the smaller Y chromosome, which in humans only contains 55 genes, a mere fraction of the roughly 1000 genes found on the X chromosome. In contrast with all the other pairs of chromosomes, for which mammals have 2 copies of each gene, the number of copies of genes carried by the sex chromosomes varies: XY individuals will have a single copy of most X-linked genes while XX individuals will have two copies of each gene.
In general, having extra or missing chromosomes can be very harmful for a cell. Having two copies of the X chromosome means a double dose of activity for genes from the X chromosome compared to XY cells. Amazingly, XX individuals utilize an epigenetic mechanism to correct this imbalance! During embryonic development, cells carrying two X chromosomes will turn off one copy – a process referred to as “X-chromosome inactivation” (XCI) – while the other copy remains active.

X-chromosome inactivation (XCI)
XCI is a classic example of an epigenetic mechanism: it does not change the DNA sequence itself but affects how genes are interpreted by the cell, and this change is transmitted to the daughter cells. The process of XCI is also named “lyonization” after Mary F. Lyon, the geneticist who first discovered this phenomenon.
To understand XCI, it is important to first remember that DNA is not “naked” in the nucleus of our cells. It is wrapped around a set of proteins called histones, much like thread around a bobbin. The addition or removal of small chemical modifications to these histones or the DNA can impact the activity of the genes nearby. These modifications are a pillar of epigenetic regulation.
The process of XCI is initiated by the production of a specific RNA called XIST, which coats the X chromosome. XIST recruits in turn the epigenetic machinery, which will add the modifications to the histone proteins located on the targeted X chromosome. These modifications make the X chromosome curl itself up and condense into a tightly packaged structure. The result is a compact mass, called the “Barr body”, which is mostly inactive. And with one X chromosome now silenced, the problem of the toxic double dose of X-linked genes is solved.
XCI happens very, very early in development. When the process begins, embryonic cells will randomly choose one of the two X chromosomes to inactivate (Figure 2). As not every cell turns off the same chromosome, the embryo becomes a cellular mosaic: the paternal copy is switched off in some cells, while the maternal copy is silenced in other cells. And once the choice is made, it is stably maintained in all daughter cells through cell division. Therefore, this early mechanism of selection can have lifelong consequences.

The science behind cats’ colors
One of the best visualisations of cellular mosaicism by way of XCI is found in the color of cats’ fur. Genes can exist in slightly different versions and for cats there is a gene with two variants of fur color - orange or black. Tortoiseshell and calico cats both have beautifully multi-colored coats with seemingly random patches of orange or black fur, with additional white patches for the calico cats. Needless to say, the gene controlling the orange versus black pigmentation of the fur is in fact found on the X chromosome and therefore under the influence of XCI.
This pattern of mixed orange and black coat coloring happens exclusively in female cats, which have two X chromosomes (Figure 3). One X chromosome can carry the gene version encoding for orange coloring, and the other X chromosome the black coloring. As mentioned before, only one of these X chromosomes will be active in any given cell, depending on the random choice happening during embryonic development. For instance, cells at the base of the tail may have the paternal X chromosome active and cause orange coloring, while cells at the end of the tail may have the maternal X chromosome active and grow black fur.

In other words, the cat’s fur is a mosaic of colors that reflects the random X-inactivation, driven by epigenetic mechanisms, that happens during early development.
As a side note, and in contrast to the orange and black coloring, the presence of additional white patches in calico cats is not under the control of the X chromosome. This condition called “piebalding”, which causes unpigmented skin and fur, is commonly found in both XX and XY cats. For example, you have likely seen white paws on cats of various colors.
What about X inactivation in human health?
XCI provides insights that go far beyond explaining the lovely colors of our favorite pets. Notably, when diseases are caused by mutations in genes on the X chromosome (thus called ‘X-linked’), they will be subject to the same type of regulation described for the cats’ fur color. Let's examine how XCI can impact certain human diseases!
One of the most common examples of X-linked disorder is red-green color blindness (Figure 4). This condition is caused by mutations in the proteins called opsins that absorb light in the retina and help us see a range of colors. One of the opsin genes is located on the X chromosome. For XY individuals, who only have a single copy of the X chromosome, one mutation is sufficient to make a person color blind. In contrast, XX individuals have two copies of the X chromosomes, with only one copy being active in each cell. If one of those chromosomes has a color blindness mutation, about half of their cells will use the healthy version of the opsin gene, and the other half will use the mutated version. In most cases, having half of your retina cells with the right opsins is sufficient to see colors properly. Thus, color blindness is much less common in women.
It is, however, still possible for XX individuals to display color blindness even with a single X chromosome mutated. This is due to the random nature of the choice of the chromosome being turned off during embryonic development. While, on average, 50% of each X will be chosen overall, it is possible for this distribution to be skewed towards one particular copy of the chromosome. For instance, if a person happens to have 90% of the mutated X active in their body by chance, they could develop color blindness despite being XX.
Beyond color blindness, many diseases and health conditions are now known to be influenced by XCI, including forms of hemophilia, muscular dystrophy, and immunodeficiency. Thus, the silencing of the X chromosome by epigenetic means has important repercussions in regard to genetics, health, and diseases.
Learn more:
- Review on XCI for a broad audience: https://kids.frontiersin.org/articles/10.3389/frym.2019.00134
- Recent insights into the molecular regulation of XCI: https://www.sciencedaily.com/releases/2022/05/220519115328.ht
- Another example of X-linked disease, hemophilia: https://openoregon.pressbooks.pub/mhccbiology102/chapter/hemophilia-a-sex-linked-disorder/
Images created in www.BioRender.com
References:
- Lyon, MF. Sex chromatin and gene action in the mammalian X-chromosome. Am. J. Hum. Genet. 1962; 14:135–48.
- Loda A, Collombet S & Heard E. Gene regulation in time and space during X-chromosome inactivation. Nat Rev Mol Cell Biol. 2022; 23(4):231-249. doi: 10.1038/s41580-021-00438-7.
- Pessia E, Makino T, Bailly-Bechet M, McLysaght A & Marais G.A.B. Mammalian X chromosome inactivation evolved as a dosage-compensation mechanism for dosage-sensitive genes on the X chromosome. Proc. Natl Acad. Sci. USA. 2012; 109, 5346–5351.
- Brown CJ et al. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992; 71, 527–542.
- Dixon-McDougall T & Brown C. The making of a Barr body: the mosaic of factors that eXIST on the mammalian inactive X chromosome. Biochem Cell Biol. 2016; 94(1):56-70. doi: 10.1139/bcb-2015-0016.
- Augui S, Nora EP & Heard E. Regulation of X-chromosome inactivation by the X-inactivation centre. Nat. Rev. Genet. 2011; 12, 429–442.
- Malcom K. How one of the X chromosomes in female embryonic stem cells is silenced ScienceDaily. 2022. https://www.sciencedaily.com/releases/2022/05/220519115328.htm.
- Cloutier M, Kumar S, Buttigieg E, Keller L, Lee B, Williams A, et al. Preventing erosion of X-chromosome inactivation in human embryonic stem cells. Nature Communications. 2022; 13. doi: 10.1038/s41467-022-30259-x
- Pereira G & Dória S. X-chromosome inactivation: implications in human disease. J Genet. 2021; 100:63.
- Singer-Sam J. Monoallelic Expression. Nature Education. 2010; 3(3):1. https://www.nature.com/scitable/topicpage/monoallelic-expression-881327….
- Niemi S & Wu H. X Marks the Spot: How X Chromosome Inactivation Gives Females an Advantage. Front. Young Minds. 2019; 7:134. doi: 10.3389/frym.2019.00134.
- Slutz S. 2019. X-inactivation Marks the Spot for Cat Coat Color. Science Buddies. https://www.sciencebuddies.org/science-fair-projects/project-ideas/MamB….